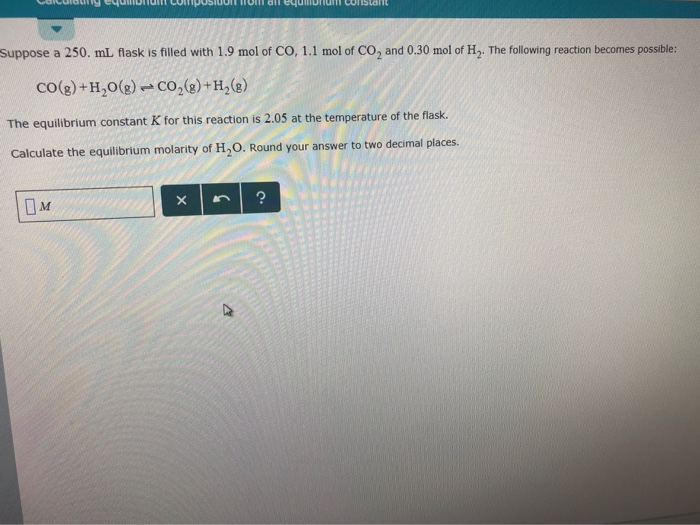
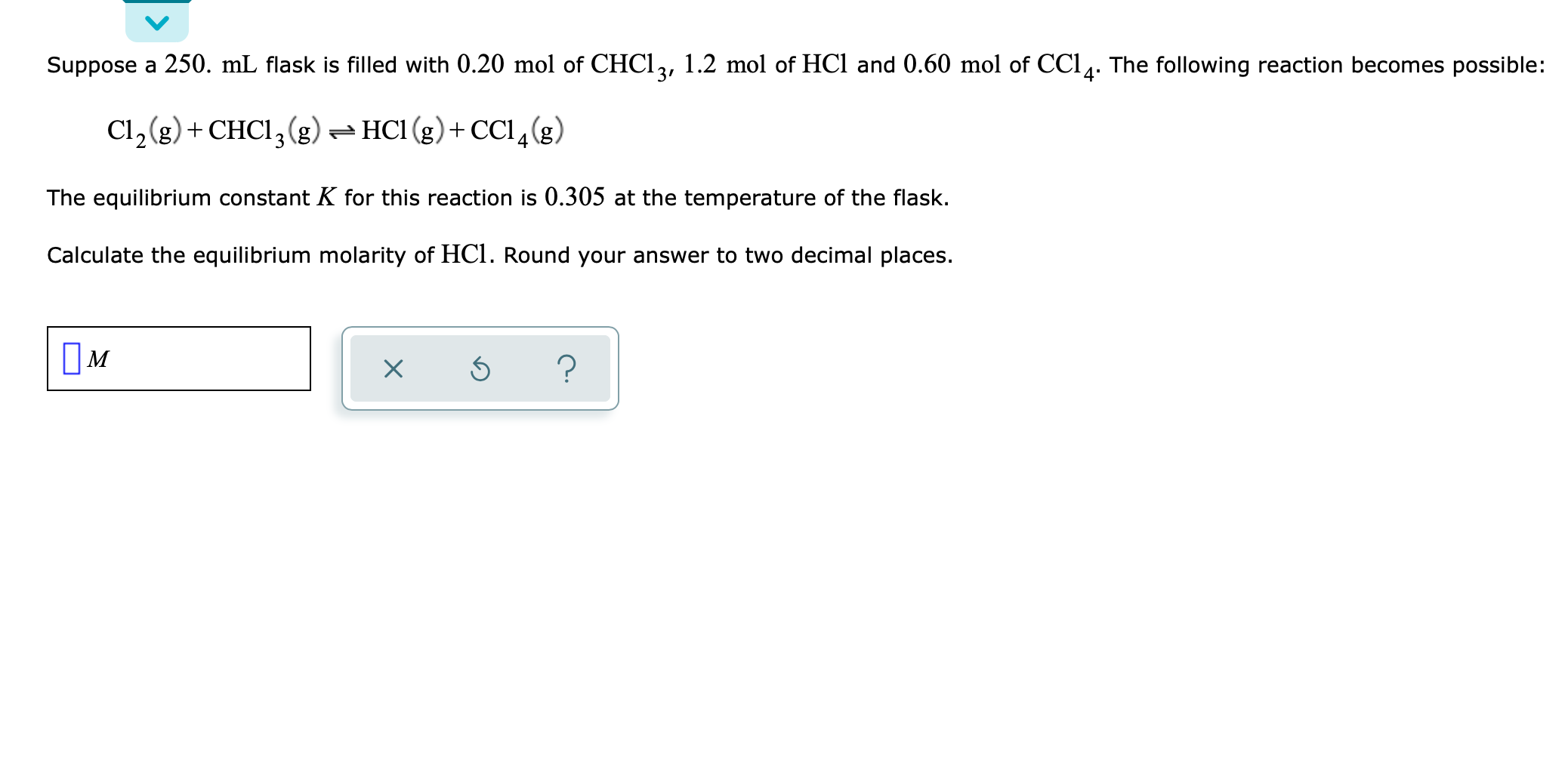
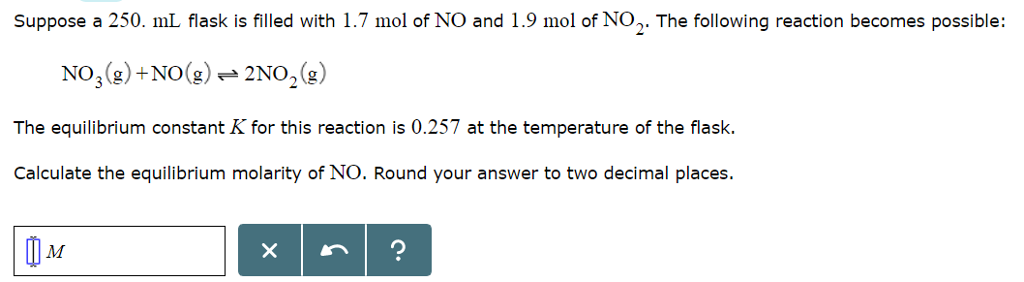
Consider the flasks in the following diagrams. Which is greater, the initial pressure of helium or initial pressure of neon? How much greater? | Homework.Study.com

Chem ch 5 and 6 HW complete.docx - Chem Ch 5 Hw A 1.07 g sample of a Noble gas occupies a volume of 363 mL at 35°C and 678 mmHg. Identify the Noble gas | Course Hero
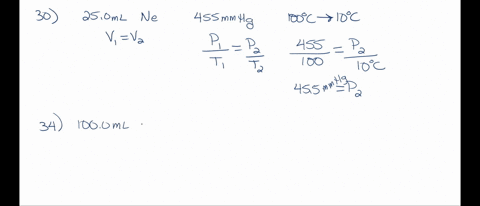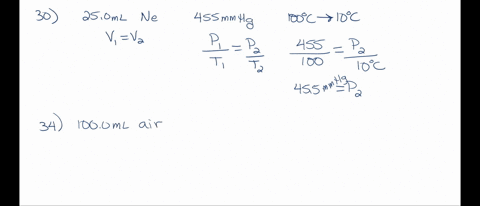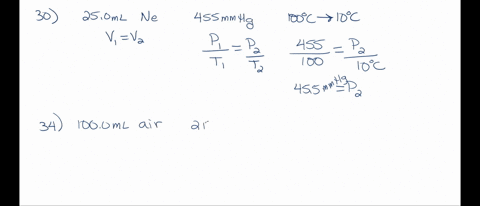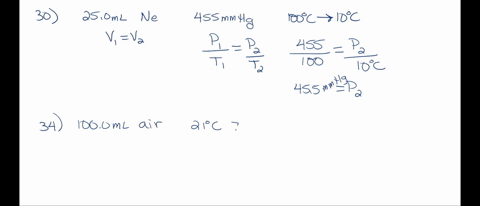
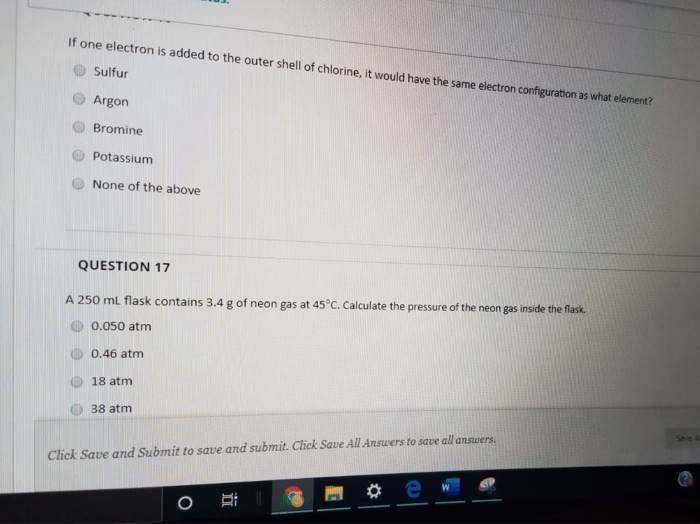
SOLVED: A 250.0 mL flask contains 3.4 g of argon gas at 45.0°C. Calculate the pressure of the neon gas inside the flask: (R = 0.0821 L·atm/mol·K; Use 273.15 for conversion to

SOLVED: A 250.0 mL flask contains 3.4 g of argon gas at 45.0°C. Calculate the pressure of the neon gas inside the flask: (R = 0.0821 L·atm/mol·K; Use 273.15 for conversion to