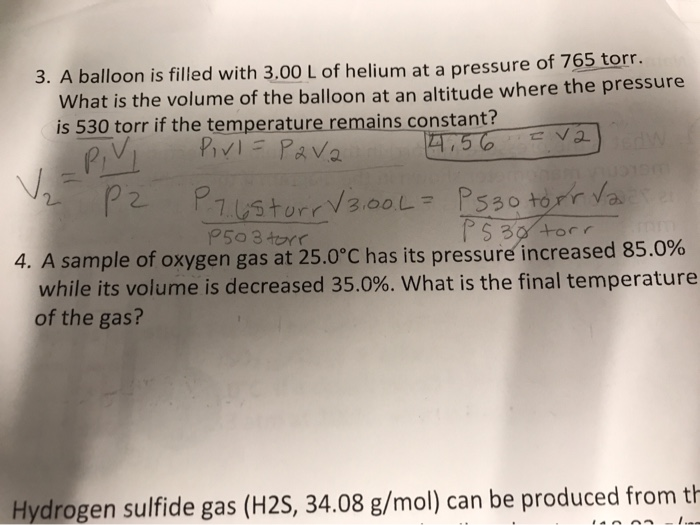SOLVED: A balloon is filled with 3.60 L of H2 gas at STP. If the balloon is taken into a sea where the pressure is 2.50 atm and the temperature is 10.00

A balloon partially filled with helium has a volume of `30 m^3`, at the earth\'s surface, where ... - YouTube
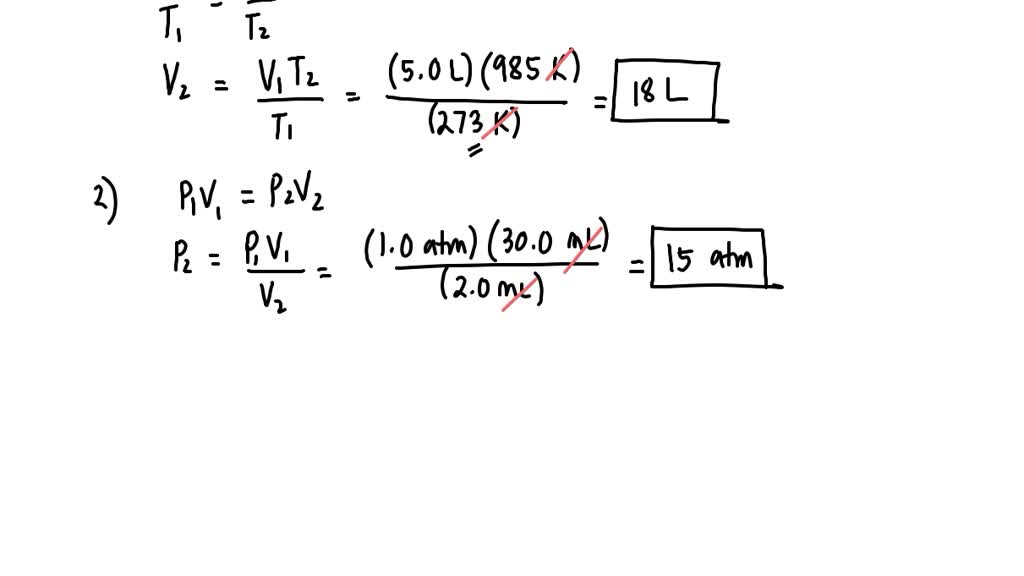
SOLVED: If 5.0 liters of H2(g) at STP is heated to a temperature of 985K, with pressure remaining constant, what will be the new volume of the gas?

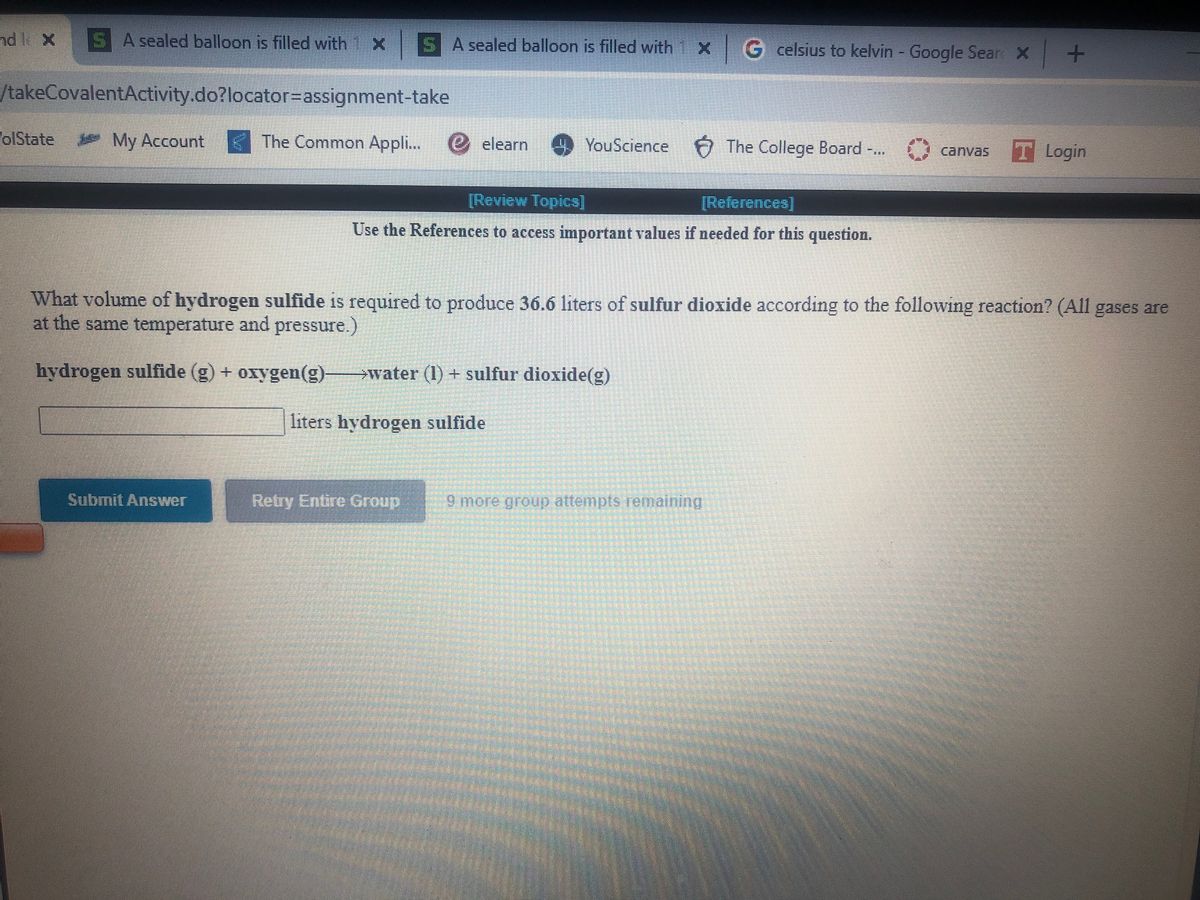
SOLVED:A balloon filled with H2(g) at 0.0^∘ C and 1.00 atm has a volume of 2.24 L. What is the final gas volume if 0.10 mol He(g) is added to the balloon
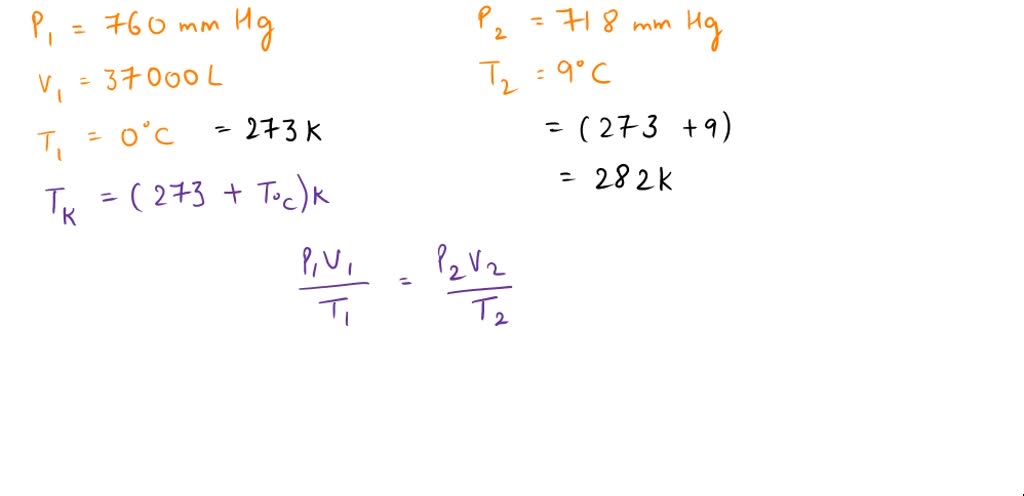
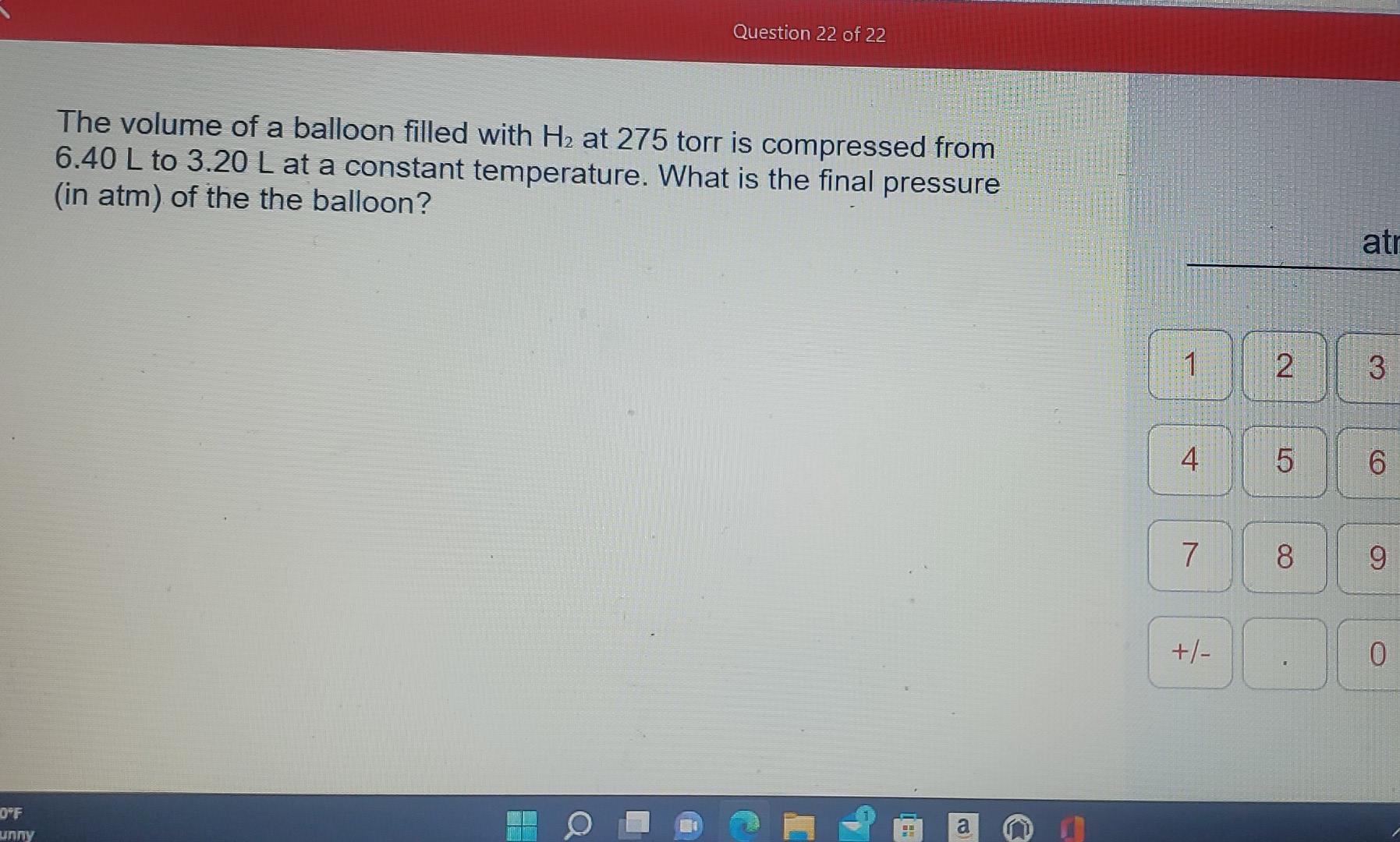
SOLVED: A balloon filled with hydrogen gas had a volume of 37000 L at a pressure of 760 mmHg and a temperature of 0 °C. When the balloon reached an altitude of

Aballoon is filled with 3.60 L of H2 gas at STP. If the balloon is taken into a sea where the pressure is - Brainly.com
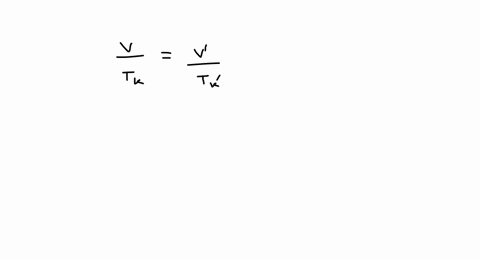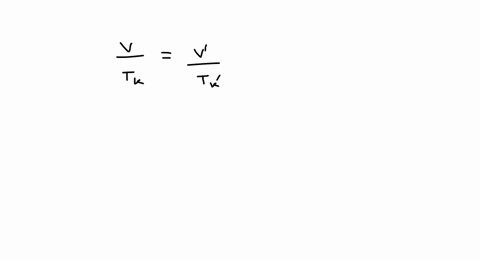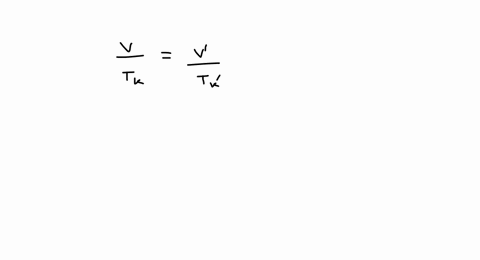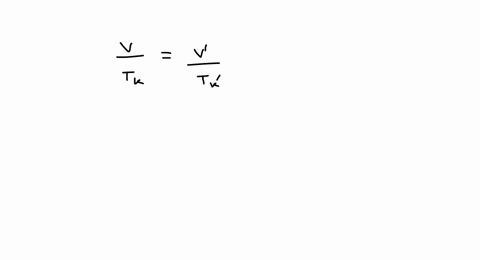
SOLVED: A balloon is filled with 3.60 L of H2 gas at STP. If the balloon is taken into a sea where the pressure is 2.50 atm and the temperature is 10.00