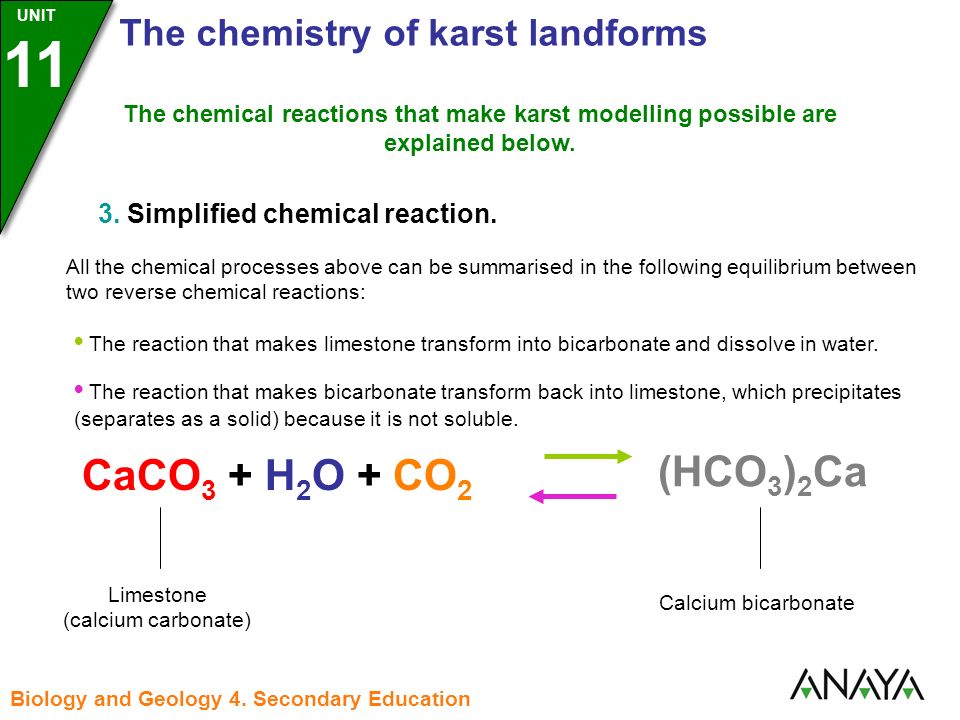
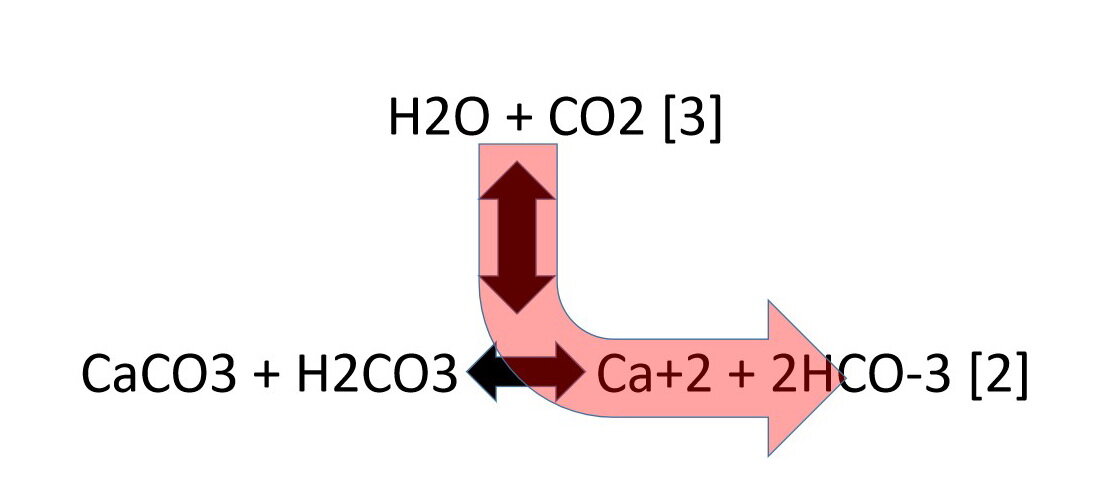
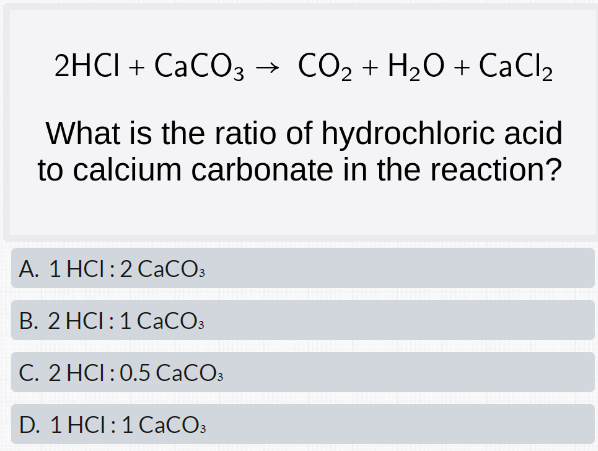
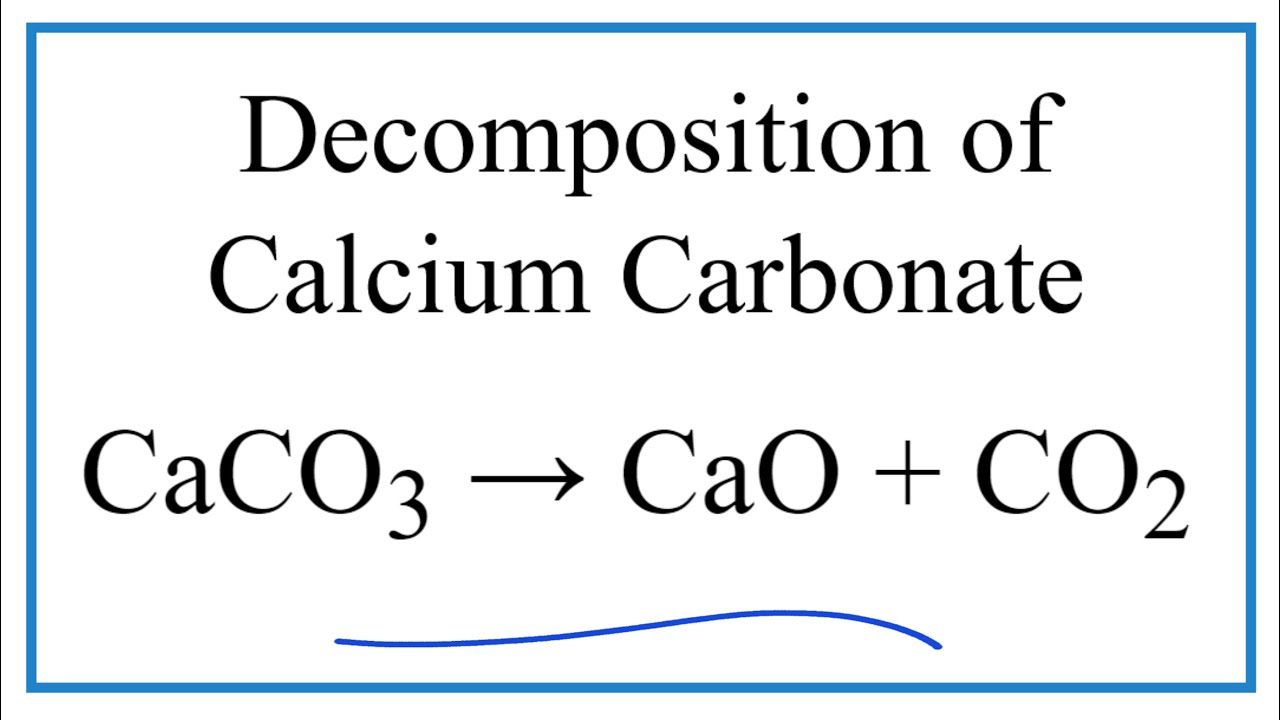
CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions - ScienceDirect
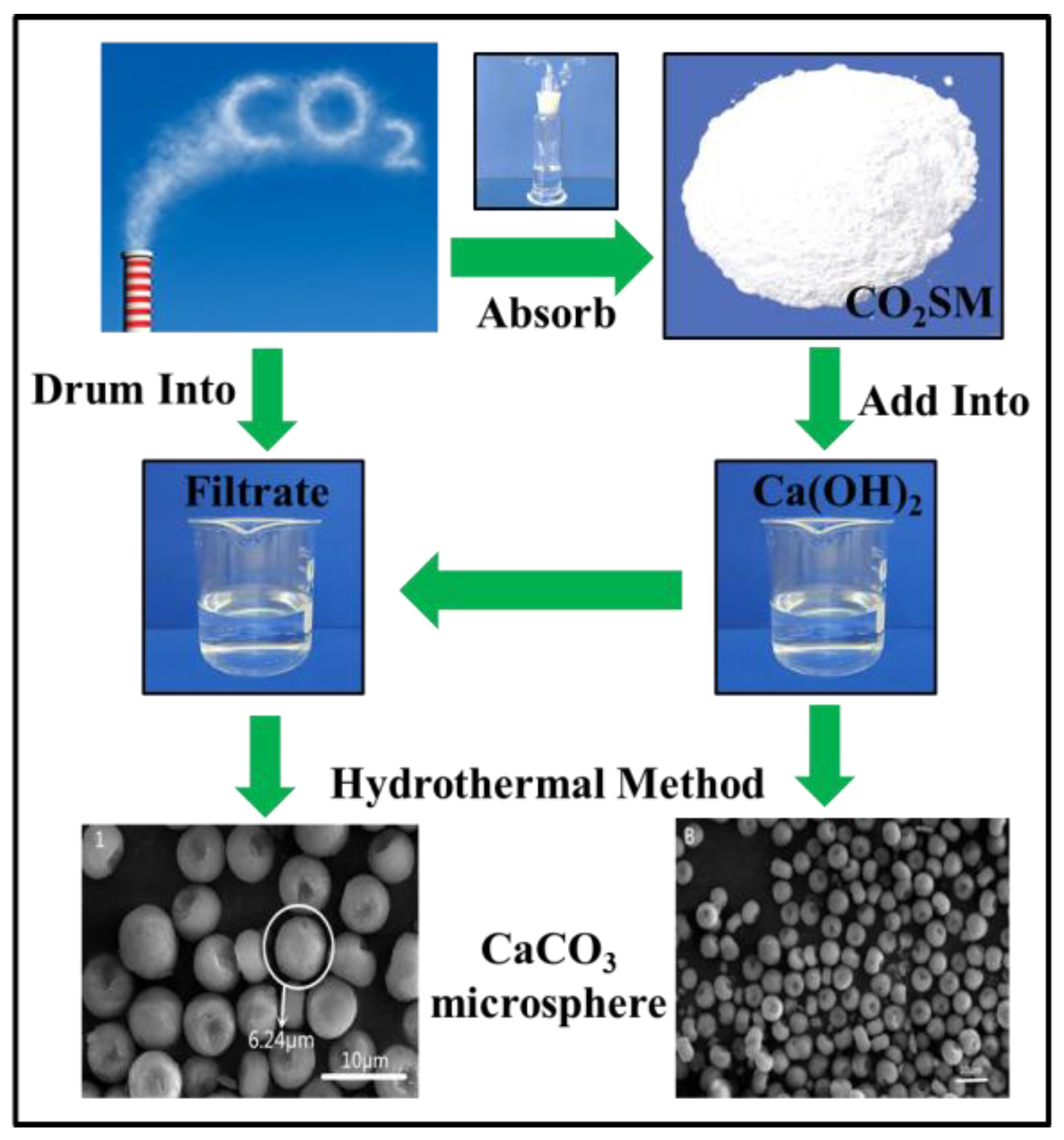
Crystals | Free Full-Text | Utilization of a CO2 Storage Material: Shape-Controlled Preparation of CaCO3 Microspheres
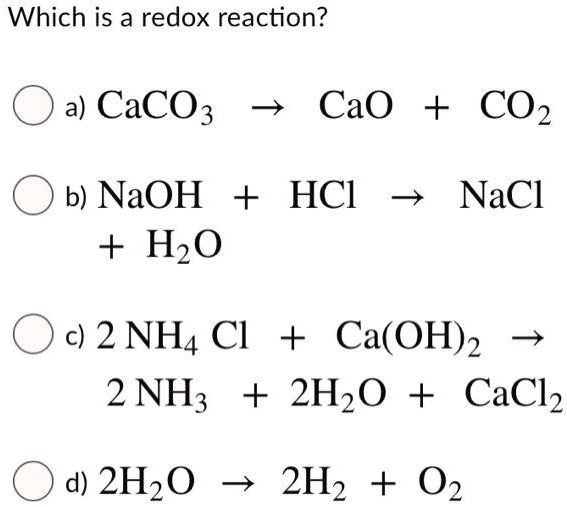
SOLVED: Which is a redox reaction? a) CaCO3 â†' CaO + CO2 b) NaOH + H2O â†' HCl + NaCl c) 2 NH4Cl + Ca(OH)2 â†' 2 NH3 + 2H2O + CaCl2 d) 2H2O â†' 2H2 + O2
CaO(s) + CO2(g) → CaCO3(s) + heat What is the total mass of CO2(s) needed to produce 300. grams of CaCO3(s)? - Quora

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa

CaCO3(s) CaO(s) + CO₂(g) 3.25 mol CaCO3 decomposes according to the reaction above. What volume of CO2 gas - brainly.com

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram
![SOLVED: What is the expression for the equilibrium constant? CaO(s) + CO2(g) ⇌ CaCO3(s) [CaO][CO2] / [CaCO3] b) Keq = [CaCO3] / [CaO][CO2] c) Keq = [ CO2] [CO2] Keq = [CO2] Keq = [ SOLVED: What is the expression for the equilibrium constant? CaO(s) + CO2(g) ⇌ CaCO3(s) [CaO][CO2] / [CaCO3] b) Keq = [CaCO3] / [CaO][CO2] c) Keq = [ CO2] [CO2] Keq = [CO2] Keq = [](https://cdn.numerade.com/ask_images/928a15231b094c709901e0e2efd39d00.jpg)
SOLVED: What is the expression for the equilibrium constant? CaO(s) + CO2(g) ⇌ CaCO3(s) [CaO][CO2] / [CaCO3] b) Keq = [CaCO3] / [CaO][CO2] c) Keq = [ CO2] [CO2] Keq = [CO2] Keq = [
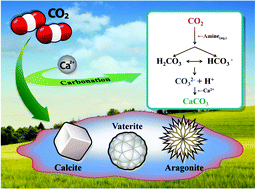
CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system - RSC Advances (RSC Publishing)

How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube