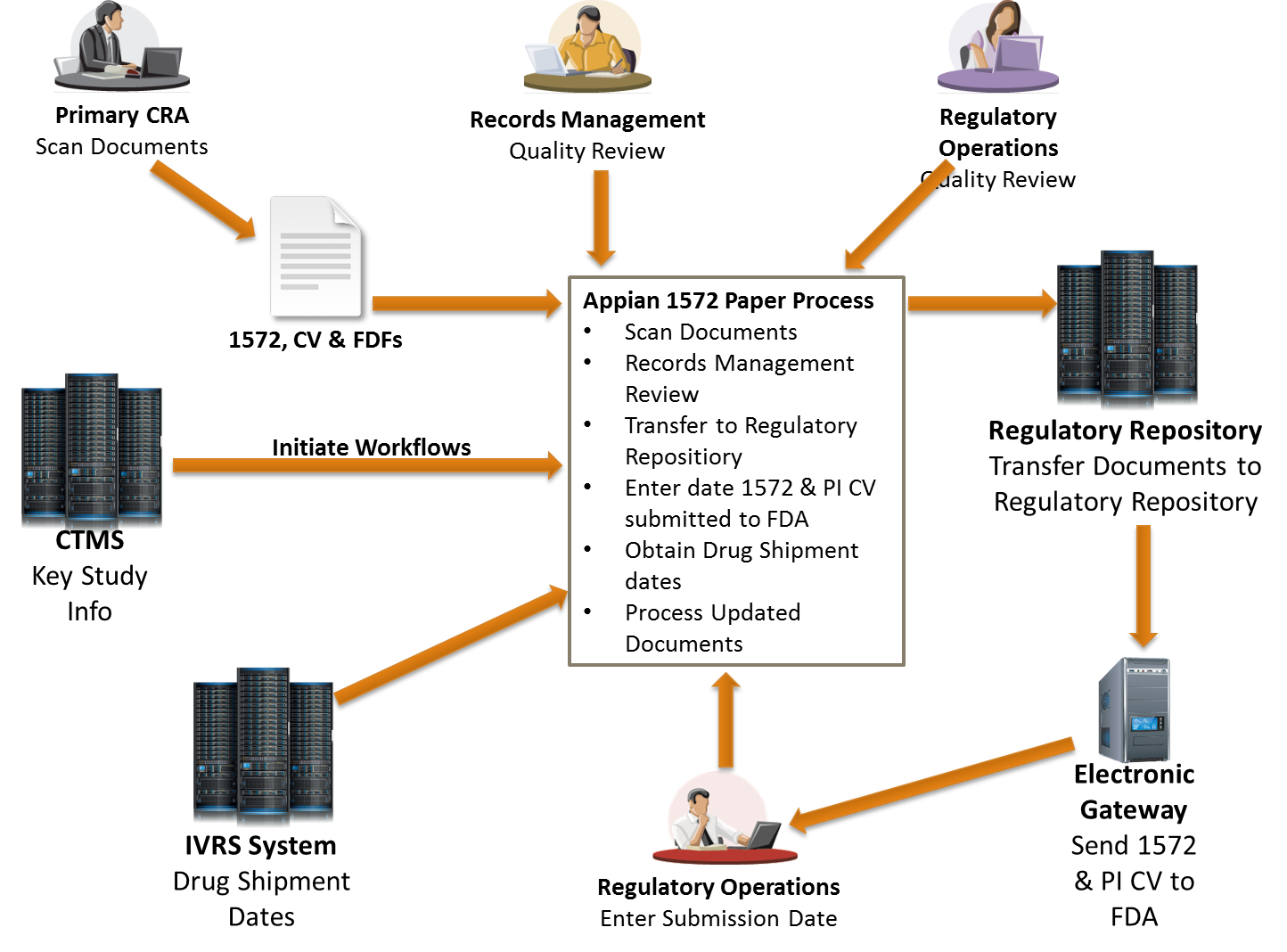
Compliance4alllearning - Form FDA 1572 is one of the primary documents needed when carrying out a clinical trial. Also called the Statement of Investigator; Form FDA 1572, called just 1572 informally, is
Form FDA 1572 Instructions General Information and Instructions This form instruction is to assist clinical investigators in com

FDA Regulatory Control of the Drug Development Process and Investigator Responsibility in the Proces by Martin Lurthur - Issuu

Fillable Online Form FDA 1572 - TransCelerate BioPharma Inc. TransCelerate ... Fax Email Print - pdfFiller